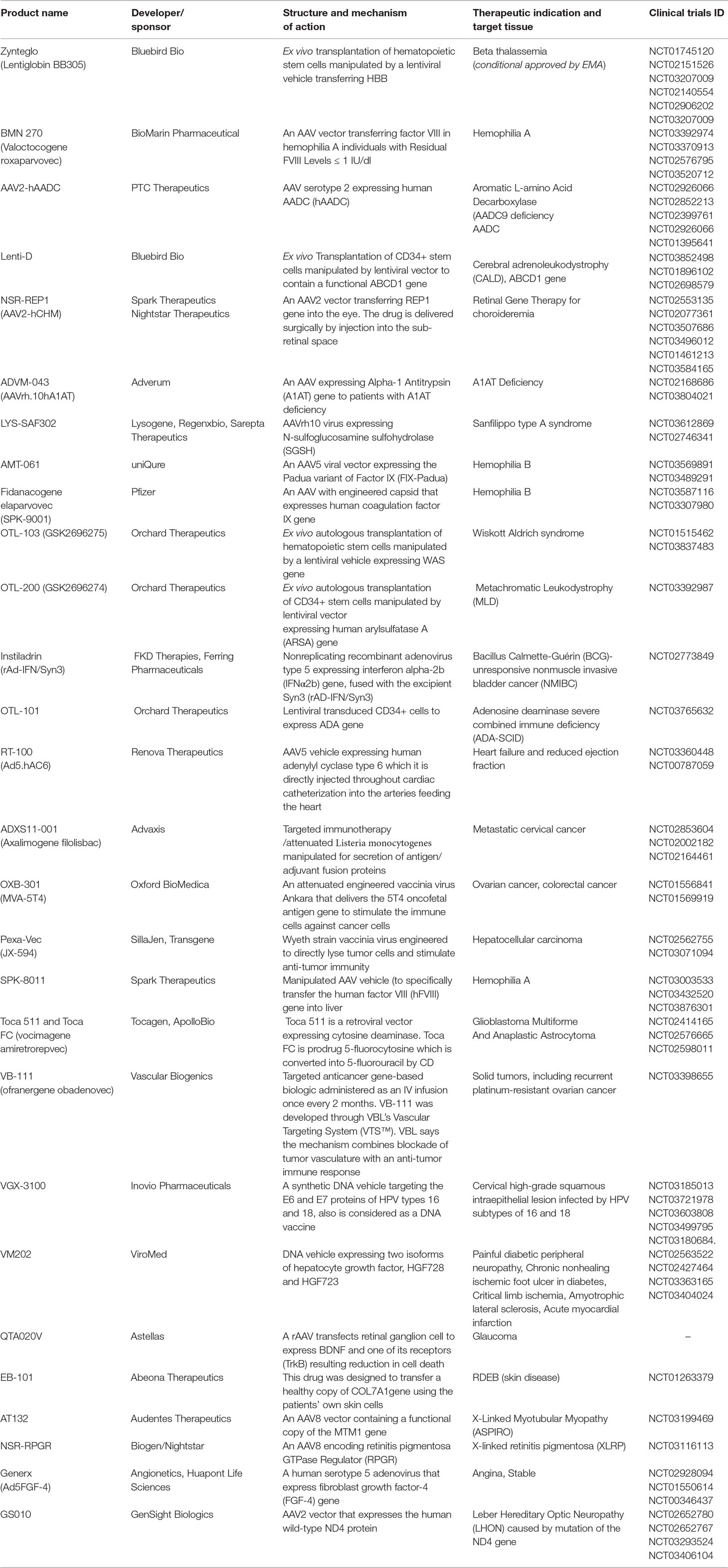
Kymriah Approval Letter. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Date hospitals were able to complete rems certification. Date rems call center operational c. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Date kymriah rems website went live b. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Novartis will continue to build a global. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. 15:40 johns hopkins medicine 3 014 просмотров. Kymriah faq with patrick brown, m.d. See full prescribing information for kymriah. Supplied by novartis pharmaceuticals corporation. Kymriah is a medicine for treating two types of blood cancer:
Kymriah Approval Letter: Kymriah™ (Tisagenlecleucel) Suspension For Intravenous Do Not Administer Kymriah To Patients With Active Infection Or Inflammatory Disorders.
Why The Fda Approved Kymriah A Car T Cell Therapy To Treat Cancer Business Insider. Date hospitals were able to complete rems certification. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Date rems call center operational c. 15:40 johns hopkins medicine 3 014 просмотров. See full prescribing information for kymriah. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Date kymriah rems website went live b. Novartis will continue to build a global. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Kymriah is a medicine for treating two types of blood cancer: Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Supplied by novartis pharmaceuticals corporation. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Kymriah faq with patrick brown, m.d.
It's only fair to share… fda approval brings first gene therapy to the united states 08/30/2017 the u.s.
The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Dear parent or guardian, your child has been selected at random to. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Kymriah faq with patrick brown, m.d. Approval letters are generally written in response to a request made by an employee of the organization for a project. Approval letters are letters written to show that a person has officially agreed to something or there are many reasons for which approval letters may be written. Arnold, we would like to thank you for your letter of approval for parents. Here's how to write an approval request with samples to help you compose your own. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; See full prescribing information for kymriah. Supplied by novartis pharmaceuticals corporation. See full prescribing information for kymriah. Novartis obtains fda approval for second indication of kymriah. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. The mpnp approval letter is an official government document. Find lots of sample letter and email format for quick uses. Date rems call center operational c. Make copies, for your personal records and for you to you must include a copy of your mpnp approval letter in your pr visa application. It's only fair to share… fda approval brings first gene therapy to the united states 08/30/2017 the u.s. Kymriah is a medicine for treating two types of blood cancer: Learn what is approval letter and how to write request for pre approval email or letter for bank home loan, mortgage and leave sanction. An approval letter can be written for a number of reasons. Date kymriah rems website went live b. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Some of the common ones include. The employer first checks in detail whether the requests in the letter are. 15:40 johns hopkins medicine 3 014 просмотров. Food and drug administration issued a historic action today making the first gene therapy. Date hospitals were able to complete rems certification. When approval is required, you might create a request for approval letter.
Oxford Biomedica Extends Lentiviral Deal With Swiss Giant, Date Hospitals Were Able To Complete Rems Certification.
Gene Therapy Drug Kymriah Should Be Publicly Covered If Manufacturer Drops Price Report Recommends The Globe And Mail. Date rems call center operational c. See full prescribing information for kymriah. 15:40 johns hopkins medicine 3 014 просмотров. Date hospitals were able to complete rems certification. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Supplied by novartis pharmaceuticals corporation. Date kymriah rems website went live b. Kymriah faq with patrick brown, m.d. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Novartis will continue to build a global. Kymriah is a medicine for treating two types of blood cancer: Kymriah uses a patient's own immune cells and reengineers them to fight cancer.
The Cancer Letter On Twitter Novartis Kymriah Gets Second Fda Approval For Large B Cell Lymphoma Https T Co Xmqujjihur : Here's How To Write An Approval Request With Samples To Help You Compose Your Own.
Zolgensma And Gene Therapy What Is The Cost Summit Re. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Date kymriah rems website went live b. Supplied by novartis pharmaceuticals corporation. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Date hospitals were able to complete rems certification. Date rems call center operational c. Kymriah is a medicine for treating two types of blood cancer: 15:40 johns hopkins medicine 3 014 просмотров. See full prescribing information for kymriah. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders.
Sec Filing Meiragtx : When approval is required, you might create a request for approval letter.
Gene Therapy S Renaissance. Supplied by novartis pharmaceuticals corporation. Novartis will continue to build a global. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Date rems call center operational c. Date hospitals were able to complete rems certification. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Kymriah is a medicine for treating two types of blood cancer: The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. 15:40 johns hopkins medicine 3 014 просмотров. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Kymriah faq with patrick brown, m.d. See full prescribing information for kymriah. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Date kymriah rems website went live b. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders.
The Car T Cell Story Open Access Healthbook , Kymriah™ (Tisagenlecleucel) Suspension For Intravenous Do Not Administer Kymriah To Patients With Active Infection Or Inflammatory Disorders.
Current State Of U S Food And Drug Administration Regulation For Cellular And Gene Therapy Products Potential Cures On The Horizon Sciencedirect. Date rems call center operational c. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; 15:40 johns hopkins medicine 3 014 просмотров. See full prescribing information for kymriah. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Date hospitals were able to complete rems certification. Kymriah faq with patrick brown, m.d. Novartis will continue to build a global. Kymriah is a medicine for treating two types of blood cancer: Date kymriah rems website went live b. Supplied by novartis pharmaceuticals corporation. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy.
Kymriah Tisagenlecleucel Official Patient Website : See Full Prescribing Information For Kymriah.
The Cost Of Gene Therapy For Patients Goes Well Beyond The Drug S Price Medcity News. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Date kymriah rems website went live b. Date hospitals were able to complete rems certification. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. 15:40 johns hopkins medicine 3 014 просмотров. Novartis will continue to build a global. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Kymriah is a medicine for treating two types of blood cancer: Kymriah faq with patrick brown, m.d. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Supplied by novartis pharmaceuticals corporation. Date rems call center operational c. See full prescribing information for kymriah.
Growth Of 4 9 Forecast For Global B Cell Nhl Market To 2027 , Some Of The Common Ones Include.
Discoveries In Health Policy Medicare S Decision On Car T Coverage No More Than 30 Days Away. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. 15:40 johns hopkins medicine 3 014 просмотров. See full prescribing information for kymriah. Date rems call center operational c. Date hospitals were able to complete rems certification. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Kymriah faq with patrick brown, m.d. Date kymriah rems website went live b. Supplied by novartis pharmaceuticals corporation. Novartis will continue to build a global. Kymriah is a medicine for treating two types of blood cancer: Kymriah uses a patient's own immune cells and reengineers them to fight cancer.
Novartis Cancer Treatment Kymriah Gets Japan Nod At Cost Of 305 800 Business Insider : See Full Prescribing Information For Kymriah.
F D A Approves First Gene Altering Leukemia Treatment Costing 475 000 The New York Times. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Date rems call center operational c. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; See full prescribing information for kymriah. Date kymriah rems website went live b. Supplied by novartis pharmaceuticals corporation. 15:40 johns hopkins medicine 3 014 просмотров. Date hospitals were able to complete rems certification. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Kymriah faq with patrick brown, m.d. Kymriah is a medicine for treating two types of blood cancer: Novartis will continue to build a global.
Novartis Kymriah Exec To Lead Atara - Approval Letters Are Generally Written In Response To A Request Made By An Employee Of The Organization For A Project.
Jcm Free Full Text Chimeric Antigen Receptor T Cells The Future Is Now Html. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Date rems call center operational c. Date kymriah rems website went live b. Supplied by novartis pharmaceuticals corporation. 15:40 johns hopkins medicine 3 014 просмотров. Kymriah faq with patrick brown, m.d. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Novartis will continue to build a global. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; See full prescribing information for kymriah. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Date hospitals were able to complete rems certification. Kymriah is a medicine for treating two types of blood cancer:
Car T Cell Therapy Will First To Market Mean First To Disappoint Seeking Alpha : Kymriah™ (Tisagenlecleucel) Suspension For Intravenous Do Not Administer Kymriah To Patients With Active Infection Or Inflammatory Disorders.
Gene Therapies Show Promise For Pediatric Treatment. Date rems call center operational c. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Date hospitals were able to complete rems certification. Novartis will continue to build a global. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Supplied by novartis pharmaceuticals corporation. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; See full prescribing information for kymriah. Date kymriah rems website went live b. Kymriah is a medicine for treating two types of blood cancer: The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. 15:40 johns hopkins medicine 3 014 просмотров. Kymriah faq with patrick brown, m.d.
Top Regulatory News Stories Week Ending May 4 2018 Dennis Partners , Dear Parent Or Guardian, Your Child Has Been Selected At Random To.
Regulatory Deep Dive On The Celgene Cvr Nyse Bmy Seeking Alpha. Novartis will continue to build a global. 15:40 johns hopkins medicine 3 014 просмотров. See full prescribing information for kymriah. Date rems call center operational c. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Date hospitals were able to complete rems certification. Kymriah faq with patrick brown, m.d. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Kymriah is a medicine for treating two types of blood cancer: Date kymriah rems website went live b. Supplied by novartis pharmaceuticals corporation. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology.